This week highlighted Cochrane Review – can mushroom extract be used for adverse effects of cancer treatment.
Title: Coriolus (Trametes) versicolor mushroom to reduce adverse effects from chemotherapy or radiotherapy in people with colorectal cancer
Objectives: To assess the effects of adjunctive Coriolus versicolor (Trametes versicolor) and its extracts on adverse effects and on survival during colorectal cancer treatment (chemotherapy and radiotherapy) compared with no adjunctive treatment.
Main results: 7 randomized trials with 1,569 participants were included.
In this publication, the authors conducted a comprehensive Cochrane systematic review with meta-analyses. Participants were diagnosed with cancer ranging from stage II to stage IV. Coriolus was used in the form of an extract in all seven studies and was generally used after curative resection, although in one study it was used preoperatively.
The rigorous analysis revealed that
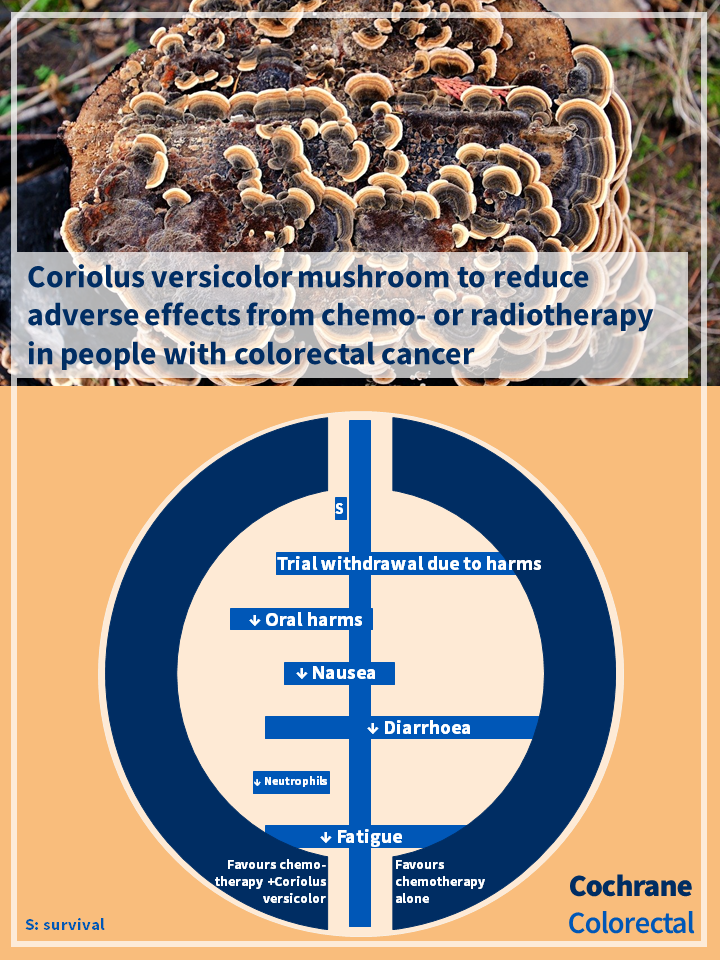
- There was little to no effect of adjunctive treatment with Coriolus on withdrawal from treatment due to adverse events, very low‐certainty evidence.
- It is uncertain whether adjunctive Coriolus versicolor and its extracts compared to usual care alone resulted in a difference in adverse events including neutropenia, oral cavity disorders such as oral dryness and mucositis, nausea, diarrhoea, and fatigue.
- There was low‐certainty evidence of a small effect of adjunctive Coriolus on improved survival at five years compared with no adjunctive care. The effect at earlier time points was unclear.
The authors concluded that due to the very low certainty of evidence, they were uncertain about the effect of adjunctive Coriolus on adverse events resulting from conventional chemotherapy for colorectal cancer. The uncertainty in the evidence also means that it was unclear whether any adverse events were due to the chemotherapy or to the extract itself. While there was low‐certainty evidence of a small effect on overall survival at five years, the influence of reduced adverse effects on this could not be determined.
Access publication here: https://doi.org/10.1002/14651858.CD012053.pub2
Congratulations to the authors:
Karen Pilkington, L Susan Wieland, Lida Teng, Xin Yan Jin, Dawn Storey, Jian Ping Liu